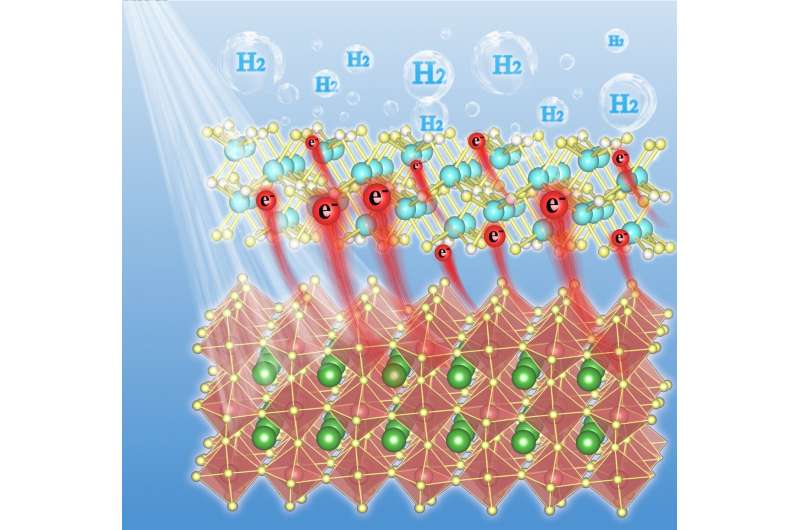
Halide perovskites have been emerging as promising photocatalytic materials for H2-evolution from water due to their outstanding photoelectric properties. However, the lack of proper surface reactive sites greatly hinders the photocatalytic potential of these fascinating compounds. In this work, Mo2C nanoparticles have been anchored onto methylammonium lead iodide (MAPbI3) as a non-noble-metal cocatalyst to promote H2-evolution reactions.
The team published their work on May. 28 in Energy Material Advances.
"Photocatalytic water splitting has been considered as a promising route to store solar energy as hydrogen energy," said paper author Xiaoxiang Xu, professor with the Shanghai Key Lab of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University. "Currently, halide perovskites have been gained great interest as photocatalysts for H2-evolution reactions, but they are normally deficient in surface reactive sites where photocarriers cannot be promptly transferred for surface redox reactions."
Xu explained that the extremely acidic environment needed to stabilize halide perovskites in an aqueous solution restrains the choices of H2-evolution cocatalyst that can be deposited.
"The noble-metal cocatalyst Pt has been introduced to promote H2-evolution reactions but is still unsatisfactory probably due to the poor Pt/halide perovskite interfaces," Xu said. "Robust non-noble-metal-based cocatalysts have gained serious attention as alternatives to noble-metal cocatalyst."
However, their connections with halide perovskites are generally very weak often due to structural mismatch thereby preventing fast charge migrations from halide perovskites to these cocatalysts. According to Xu, Mo2C, a promising low-cost electrocatalyst for H2-evolution reaction, exhibits excellent electrocatalytic activity over a wide pH range thereby serving as a potential candidate cocatalyst for photocatalytic H2-evolution.
Mo2C is one of a few compounds that are stable in strong acids, Xu said, rendering it an excellent alternative cocatalyst for halide perovskites which are stabilized in strong acid during photocatalytic reactions, e.g. HBr and HI aqueous solution.
"The opposite Zeta potentials between Mo2C and MAPbI3 ensure firm interconnections between these compounds," Xu said. "In this paper, we illustrated recent research expediting H2-evolution over MAPbI3 with a non-noble metal cocatalyst Mo2C under visible light."
"We have successfully loaded Mo2C nanoparticles onto MAPbI3 by an electrostatic-assembly method to fabricate Mo2C@MAPbI3 composites," Xu said.
"The Mo2C nanoparticles are found to be homogeneously and firmly anchored at the surface of MAPbI3," Xu said. "Thanks to the strong interconnections between Mo2C and MAPbI3, Mo2C@MAPbI3 composites exhibit superior photocatalytic activity for H2-evolution from water which clearly surpasses pristine MAPbI3 and Pt deposited MAPbI3 under the same testing conditions."
"Further analysis suggests that Mo2C nanoparticles not only facilitate charge separation in MAPbI3 but also substantially expedite interfacial charge transfer for water reduction reactions," Xu said. "These findings justify the Mo2C as an efficient non-noble-metal cocatalyst for halide perovskite photocatalysts that work under a highly acidic environment."
Provided by Beijing Institute of Technology Press
Citation: Expediting hydrogen creation with a non-noble metal cocatalyst under visible light (2022, June 2) retrieved 2 June 2022 from https://ift.tt/VaoGsnR
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"creation" - Google News
June 02, 2022 at 11:55PM
https://ift.tt/VaoGsnR
Expediting hydrogen creation with a non-noble metal cocatalyst under visible light - Phys.org
"creation" - Google News
https://ift.tt/RUZEpob
https://ift.tt/BzEFVsW
Bagikan Berita Ini














0 Response to "Expediting hydrogen creation with a non-noble metal cocatalyst under visible light - Phys.org"
Post a Comment